Timeline Industrial Revolution and the 20th century
1788
- First Fleet arrives in Australia
-
 Because of its island nature the Australian continent and its ecosystems had been develop in relative isolation. That changed when humans arrived, first about 60,000 years ago and then with the arrival of the first convict fleet in Sydney Harbour in 1788. In the word of historian George Seddon: "[Australia] had a radically new technology imposed upon it, suddenly, twice”. The two waves of human arrivals each brought major technological shocks to the ecosystems. Aboriginal people hunted and modified the landscape with fire. The European settlement brought simultaneous agricultural and industrial revolutions which modified the Australian landscape and environments faster than anything before.
Because of its island nature the Australian continent and its ecosystems had been develop in relative isolation. That changed when humans arrived, first about 60,000 years ago and then with the arrival of the first convict fleet in Sydney Harbour in 1788. In the word of historian George Seddon: "[Australia] had a radically new technology imposed upon it, suddenly, twice”. The two waves of human arrivals each brought major technological shocks to the ecosystems. Aboriginal people hunted and modified the landscape with fire. The European settlement brought simultaneous agricultural and industrial revolutions which modified the Australian landscape and environments faster than anything before.
1850
- Plowing up the World’s Grasslands
-
One of the great transformations in global environmental history has been the plowing up of the world’s grasslands to grow grain. The process began at the western end of the Eurasian steppes, in present-day southern Russia and Ukraine, in the second half of the eighteenth century. In the nineteenth and twentieth centuries, the great plow-up spread to vast areas of the prairies and Great Plains of North America, the Pampas of South America, the veldt of southern Africa, lands in Australasia, northern India, and north Africa, and the plains of Hungary and Romania.
This process was driven by the transport revolution aided by the construction of railroads, cargo ships, ports, and grain elevators. Facilitated by domestic and international markets, the grain was consumed by the globe’s burgeoning urban population.
The conversion of large parts of the world’s grasslands to arable fields was achieved with great social and environmental cost. Indigenous populations were forcibly driven of the land and their ways of life, which were based on herding or hunting livestock, was destroyed. The Indian Wars of the late nineteenth century and wars on the Eurasian steppes, over many centuries, between states based on farming and pastoralism opened the way to the plow.
The plowing of the fertile soil to grow grain, was also followed by ecological disasters, of which the Dust Bowl on the southern plains of the USA in the 1930s is probably the most infamous.
The plowing up of the steppes is a story that is directly related to the rise of oil, nitrogen fixation as well as the transport revolution and is part of the environmental legacy of the 19th and 20th century.
1859
- The world's first mechanically drilled oil well
-
 Although the oil well at Titusville, Pennsylvania, drilled in 1859, was not the first in the world, it was the first drilled with mechanical power using a steam engine. This well symbolizes the shift away from a coal economy to an oil and gas dominated one. The rise of oil has had one of the most pronounced effects on world history. It made the automobile possible, a development that changed the face of the planet and drove urban expansion in a way never seen before. The long term consequences could even be more dramatic with the growing indications that with burning the black gold we are altering the climate of the planet. As such the triplet of energy history, transportation history and climate history, are important related areas of study for environmental historians.
Although the oil well at Titusville, Pennsylvania, drilled in 1859, was not the first in the world, it was the first drilled with mechanical power using a steam engine. This well symbolizes the shift away from a coal economy to an oil and gas dominated one. The rise of oil has had one of the most pronounced effects on world history. It made the automobile possible, a development that changed the face of the planet and drove urban expansion in a way never seen before. The long term consequences could even be more dramatic with the growing indications that with burning the black gold we are altering the climate of the planet. As such the triplet of energy history, transportation history and climate history, are important related areas of study for environmental historians.
1899
- The Invention of Mass Destruction Mining
-
With the growth of technological power in the 19th and 20th century came also a growth in applying these technologies to extract resources on scales not seen before. Oil extraction is one and open pit mining is another. Open pit, or mass destruction mining, is the process of digging away entire mountains or creating enormous pits for the extraction of metals and minerals. It leaves large scars in the landscape. By 1990 it was estimated that world open-pit mining operations moved twenty billion tons of rock each year, making it a more powerful geological force than natural erosion. If global demand for metals continues to grow, the pressure to expand the use of their mass destruction technology will likely grow with it. Although not yet part of the environmental memory of the world, it will certainly leave huge scars on the planet that will only heal with geological time.
1945
- Atomic warfare
-
Bombing of Hiroshima and Nagasaki, 1945
 The end of humanity or civilisation has been popular in gothic and proto-SF literature since the 19th century. H G Wells’ War of the Worlds is a good example of Armageddon unleased on the world population. Real world events such as the Black Death also reverberate in the environmental memory of Europe and Asia but nothing had prepared humanity for the powers that would be unleased during the 20th century.
The end of humanity or civilisation has been popular in gothic and proto-SF literature since the 19th century. H G Wells’ War of the Worlds is a good example of Armageddon unleased on the world population. Real world events such as the Black Death also reverberate in the environmental memory of Europe and Asia but nothing had prepared humanity for the powers that would be unleased during the 20th century.The mushroom clouds over Hiroshima and Nagasaki have been burned onto the retina of present day global consciousness. The introduction of nuclear weapons into warfare has to be considered one of the most important environmental events in human history with its potential to annihilate and alter life across the planet. The further development of nuclear weapons since the Second World War even threatened the very existence of the human race itself.
1950
- The great Acceleration
-
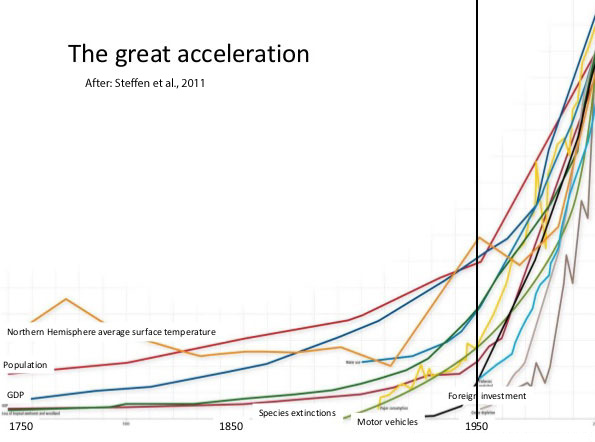
The most remarkable development of the 20th century was the "Great Acceleration", the sharp increase in human population, economic activity, resource use, transport, communication and scientific, in particular since World War II and which has continued into the 21st century. The “engine” of the Great Acceleration is an interlinked system consisting of population increase, rising consumption, abundant cheap energy, and liberalised political economies.
With the Great Acceleration came also a globalization of environmental problems. Embedded in the idea of the Great Acceleration is the J-curve, statistical graphs that turn progressively upwards. Whether it is population, economies, extinctions or numbers of cars, the story is all about growth. But this growth has its limits and beyond certain thresholds starts to impact on the global environmental systems, which are most visible as global warming and ozone depletion.
Further reading
Will Steffen, Jacques Grinevald, Paul Crutzen, and John McNeill, "The Anthropocene: conceptual and historical perspectives", Philosophical Transactions of the Royal Society of London A: Mathematical, Physical and Engineering Sciences 369, no. 1938 (2011): 842-867.
1952
- The Great London Smog
-
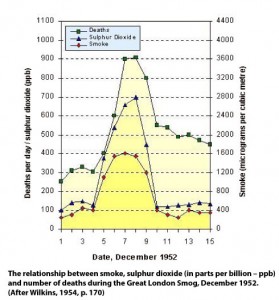 On December 4th 1952, an anticyclone settled over London. The wind dropped, the air grew damp and an inversion layer trapped the air, which received smoke from 3.5 million chimneys and many industrial smoke stacks. Smoke level rose 300 percent and the sulphur dioxide levels by 700 percent. One estimate put the pH of the water droplets as low as 1.6, the equivalent of breathing in a mist of concentrated lemon juice. Hardly surprisingly, this led to around 4000 deaths, mainly (but not exclusively) amongst the elderly and those with respiratory problems. The peak in the number of deaths coincided with the peak in both smoke and sulphur dioxide pollution levels.
On December 4th 1952, an anticyclone settled over London. The wind dropped, the air grew damp and an inversion layer trapped the air, which received smoke from 3.5 million chimneys and many industrial smoke stacks. Smoke level rose 300 percent and the sulphur dioxide levels by 700 percent. One estimate put the pH of the water droplets as low as 1.6, the equivalent of breathing in a mist of concentrated lemon juice. Hardly surprisingly, this led to around 4000 deaths, mainly (but not exclusively) amongst the elderly and those with respiratory problems. The peak in the number of deaths coincided with the peak in both smoke and sulphur dioxide pollution levels.In the aftermath of the Great London Smog of 1952, the Government pass the two Clean Air Acts of 1956 and 1968, which aimed to control domestic sources of smoke pollution by introducing smokeless zones, and control industrial sources of pollution by the use of tall chimneys for waste gas dispersal.
Although all these measures worked reasonable, the smog problem disappeared from British and other European cities due to the change in fuel. The switch to gas heating and also using gas for electricity generation made this kind of smog a thing of the past.
However, it with the rise of traffic we got a new kind of smog: photochemical smog. This kind of smog is caused by carbon hydrates which are produced by the millions of cars driving around. Carbon Hydrates react with strong sunlight and forms troposphere ozone which is poisonous and irritates eyes, nose and lungs. Cars are also causing significant increases in fine dust in the air, which is impacting negatively on people's health. These new types of air pollution are current concerns for governments and policy makers.
Further reading
Davis, Devra, ‘The Great Smog’, History Today, Vol. 52, No. 12 (2002), 2-3Wilkins, E. T., ‘Air pollution aspects of the London fog of December 1952’, Quarterly journal of the Royal Meteorological Society, 80 (1954) 344, 267-271.
1958
- David Keeling starts CO2 observations atop Mauna Loa
-
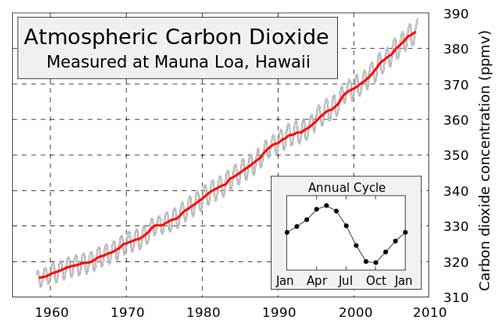
In 1958 David Keeling started his now famous measurements of the amount of CO2 in the atmosphere atop the Mauna Lowa in Hawaii. After several years of measurements it became clear that the amount of CO2 in the atmosphere was rising, even in far remote places like Mauna Loa.
The resulting graph shows the history of atmospheric carbon dioxide concentrations as directly measured at Mauna Loa, Hawaii. This curve is now known as the Keeling curve, and is an essential piece of evidence of the man-made increases in greenhouse gases that are believed to be the cause of global warming. The Mauna Loa observations are the longest record of carbon dioxide increase available and confirm that human activity is increasing the amount of this gas in the atmosphere. The annual fluctuations in the graph are caused by seasonal variations in carbon dioxide uptake by vegetation. Since more forest is concentrated in the Northern Hemisphere, more carbon dioxide is removed from the atmosphere during Northern Hemisphere summer than Southern Hemisphere summer. This annual cycle is shown in the inset figure by taking the average concentration for each month across all measured years. The black line shows the average monthly concentrations of CO2.
1970
- Earth Day
-
The first Earth Day was held on 22 April 1970. The first Earth Day reflected a major increase in public awareness of and concern about environmental problems in the US. The massive involvement of people across the United States made clear to politicians that this was an important issues and the following years there was a relatively quick succession of environmental legislation such as the clean air act and toxic substances control act.
Over time Earth day became an international event and in the process it built a lasting eco-activist-infrastructure such as lobbying organizations, environmental-studies programs, environmental beats at newspapers, and community ecology centers. Earth Day helped to give birth to the first green generation.
1972
- United Nations Conference on the Human Environment
-
The United Nations Conference on the Human Environment (also known as the Stockholm Conference) was an international conference convened by the United Nations and held in Stockholm, Sweden in June 1972. It was the UN's first major conference on international environmental issues, and marked a turning point in the development of international environmental politics putting environmental issues firmly into the global environmental consciousness. The meeting was heavily influenced by the report for the Club of Rome which predicted a resource and environmental degradation problem that would lead to a collapse of the global civilisation by 2020.
The Stockholm conference provided the blueprint for the environmental summits in Rio de Janeiro in 1992 and Johannesburg in 2002, when the attention shifted to the issue of global warming.
1974
- Stratospheric ozone depletion
-
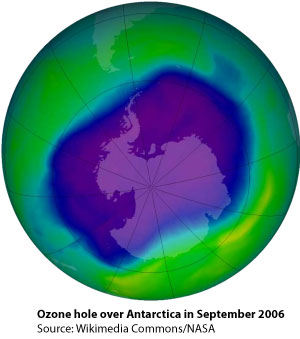 In 1974, Sherwood Rowland and Mario Molina discovered that CFCs are agents that can destroy stratospheric ozone under the influence of ultraviolet light. By 1977 it was almost certain that these gases, which were used on a large scale in spray cans and refrigerator systems, was almost certainly damaging the ozone layer, which protects us from harmful UV-B radiation. However, governments, pressurised by the chemical industry, refused to act since the mechanisms by which ozone was destroyed were by then not fully understood. It was argued that more data and research was needed to warrant action.
In 1974, Sherwood Rowland and Mario Molina discovered that CFCs are agents that can destroy stratospheric ozone under the influence of ultraviolet light. By 1977 it was almost certain that these gases, which were used on a large scale in spray cans and refrigerator systems, was almost certainly damaging the ozone layer, which protects us from harmful UV-B radiation. However, governments, pressurised by the chemical industry, refused to act since the mechanisms by which ozone was destroyed were by then not fully understood. It was argued that more data and research was needed to warrant action.By the mid-1980s a severe seasonal thinning of ozone over the Antarctic was observed and by 1987 the world’s media were reporting on a ‘Hole in the Ozone Layer’. It was during that year that the Montreal Protocol established a scheme that led to a total global ban of the production of CFCs by the late 1990s. In 2003, observed levels of chlorine in the atmosphere peaked and then began to fall. However, they will remain high for decades to come and it is expected that atmospheric concentrations of ozone will not return to natural levels before the middle of the century.
Further reading
G. Megie, ‘From stratospheric ozone to climate change: historical perspective on precaution and scientific responsibility’, Science and Engineering Ethics, 12 (2006), 596–606.
Jan, Oosthoek, 'The IPCC and the Ozone Hole: a Warning from History', Globalizations,. 5(2007), 63-66.
1986
- Chernobyl nuclear disaster
-
The nuclear disaster in Chernobyl was an event that has send shockwaves around the globe and its effects are still being felt to the present day. It was another nail in the coffin of the Soviet Union because it exposed in a dramatic fashion the political, economic and managerial failures of Communism. In addition it influenced the debate about nuclear power until the present day and has delayed a nuclear renaissance until late into the first decade of the 21st century. The memories of Chernobyl came dramatically to the fore again with the nuclear disaster at the Fukushima nuclear power plant in 2011.
1988
- Human induced climate change
-
Climate change is probably the most prominent environmental issue of today but the first signs of a warming world appeared in the late 19th century. This was caused by the industrial emissions of greenhouse gasses such as carbon dioxide (CO2) and methane (CH4). Carbon dioxide is the by product of the burning of fossil fuels such as goal, oil and gas. These energy sources, in particular coal, had fuelled the industrial revolution. During the 20th century the use of fossil fuels continued to increase and so did the emissions of carbon dioxide. This particularly accelerated since the 1950s, the period of the Great Acceleration. By the 1970s scientists became increasingly concerned about the amount of greenhouse gasses released into the atmosphere but it was not yet a public, political or economic concern.
 This changed in 1988 when NASA climatologist Dr. James Hansen testified before a US senate committee that, “The greenhouse effect has been detected and it is changing our climate now.” Hansen made his statement in June, during a heat wave in what turned out to be the hottest year on record in the continental U.S up till that point in time.
This changed in 1988 when NASA climatologist Dr. James Hansen testified before a US senate committee that, “The greenhouse effect has been detected and it is changing our climate now.” Hansen made his statement in June, during a heat wave in what turned out to be the hottest year on record in the continental U.S up till that point in time.During the same year the World Meteorological Organization and the United Nations formed a joint organization: the Intergovernmental Panel on Climate Change (IPCC). This new body was charged to fairly and openly assess the science and socio-economic challenges that societies are facing in the light of climate change. To date, the IPCC has published four full assessment reports in 1990, 1995, 2001, 2007 and the most recent one in 2013. The reports are a comprehensive review of the current state of scientific knowledge about global climate change, bringing together evidence of changes in the chemical composition of the atmosphere, evidence of warming of the climate system, understanding of the human contribution to the observed warming, and projections of changes to the global climate expected during the next few centuries.
1988 can can be regarded as the year that human induced climate change became a major public, political and environmental issue. The issue challenges our understanding of history, our vision for the future, and the nature of our species.
Recent Comments